INTRODUCTION
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) characterized by megakaryocyte proliferation and progressive bone marrow fibrosis. Additional clinical features include splenomegaly due to extramedullary hematopoiesis, cytopenias resulting from bone marrow failure, and constitutional symptoms. MF can develop de novo (primary MF) or from the progression of antecedent MPNs, in particular polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF). At diagnosis, nearly one-quarter of patients (pts) are red blood cell transfusion dependent, and nearly 40% exhibit hemoglobin levels < 10 g/dL. Additionally, pts with MF have a shortened survival and reduced quality of life (QOL).
Ruxolitinib (RUX), a first-in-class JAK1/JAK2 inhibitor, is the standard of care for MF and was approved on the basis of the COMFORT studies. In these studies, RUX demonstrated superiority over placebo (COMFORT-I) and best available therapy (COMFORT-II) in improving splenomegaly, MF-related symptoms, and QOL. An overall survival benefit was also observed with RUX (Harrison CN, et al. Leukemia. 2016). However, RUX is not a curative therapy and cytopenias remain a challenge in the management of MF. Additionally, not all pts respond to RUX, with some losing response while on treatment and some discontinuing treatment due to adverse events. RUX in combination with novel agents may offer superior disease control and transformative clinical benefits, including prolonged progression-free survival and improved cytopenias and QOL, and may reduce the malignant clone and bone marrow fibrosis.
METHODS
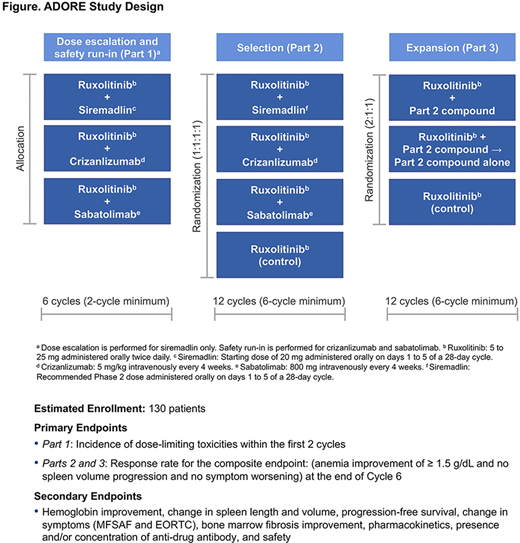
ADORE (NCT04097821) is a 3-part, open-label, multicenter, Phase 1/2 open-platform study that will assess the safety and efficacy of RUX in combination with ≥ 3 novel compounds (siremadlin [HDM2 inhibitor], crizanlizumab [P-selectin inhibitor], or sabatolimab [TIM-3 inhibitor]) for the treatment of MF (Figure).
The study will include pts ≥ 18 years old who have primary MF, PPV-MF, or PET-MF, splenomegaly (a palpable spleen ≥ 5 cm from the left costal margin or spleen volume ≥ 450 cm3 by MRI or CT scan), hemoglobin level < 10 g/dL, and platelet count ≥ 75 × 109/L (≥ 50 × 109/L in Parts 2 and 3). Pts must have been treated with RUX for ≥ 24 weeks and have received a stable dose for ≥ 8 weeks prior to study entry. Key exclusion criteria include splenic irradiation within 6 months or blood platelet transfusion within 28 days of study start.
Part 1 (Phase 1b) includes a Dose-escalation arm to determine the recommended Phase 2 dose (RP2D) for the combination of siremadlin and RUX, and 2 safety run-in arms to confirm the doses of crizanlizumab plus RUX and sabatolimab plus RUX combinations. RUX will be administered at the same stable dose used prior to study entry. A Bayesian logistic regression model will be used to identify the maximum tolerated dose and/or RP2D for the siremadlin and RUX combination. Pts will be treated for a planned duration of ≥ 6 cycles (24 weeks). The primary endpoint for Part 1 is the incidence of dose-limiting toxicities within the first 2 cycles. The combination treatments evaluated as safe and tolerable in Part 1 will proceed to Part 2.
In Part 2 (Phase 2; early efficacy assessment, Selection), pts will be randomized with equal probability to 1 of the selected combination treatments or RUX monotherapy and treated for ≥ 12 cycles (48 weeks). After all Part 2 pts have completed 24 weeks of treatment, an interim analysis will be conducted to determine which treatment should be dropped for futility or considered in Part 3 (Phase 2; efficacy, Expansion).
In Part 3, pts will be randomized 2:1:1 to the combination treatment arm, RUX cessation arm (novel agent monotherapy), or RUX monotherapy arm and treated for ≥ 12 cycles. Pts in the RUX cessation arm will be treated with RUX combination therapy for 12 weeks, followed by tapering of RUX.
The primary endpoint for Parts 2 and 3 is the response rate for the composite endpoint of anemia improvement (increase in hemoglobin of ≥ 1.5 g/dL), no spleen volume progression, and no symptom worsening at the end of 6 treatment cycles (24 weeks). Secondary efficacy endpoints include hemoglobin improvement, change in spleen size, progression-free survival, change in symptoms, and bone marrow fibrosis improvement. The study will end 24 months after the last pt has initiated Part 3. Planned enrollment for the study with the current 3 combinations is 130 pts. Enrollment is currently ongoing.
Perkins:Novartis Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lehmann:Novartis: Consultancy, Research Funding. Reiter:Incyte: Consultancy, Other: travel support ; AOP: Consultancy, Other: travel support ; Celgene,: Consultancy, Other: travel support ; Abbvie: Consultancy, Other: travel support ; Deciphera: Consultancy, Other: travel support ; Blueprint: Consultancy, Other: travel support ; Novartis: Consultancy, Honoraria, Other: travel support , Research Funding; Gilead: Other: travel support . Gupta:Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol MyersSquibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Incyte: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Harrison:Roche: Honoraria; Sierra Oncology: Honoraria; Promedior: Honoraria; AOP Orphan Pharmaceuticals: Honoraria; Celgene: Honoraria, Research Funding, Speakers Bureau; Shire: Honoraria, Speakers Bureau; CTI Biopharma Corp: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau; Incyte Corporation: Speakers Bureau; Janssen: Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Kiladjian:AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Vannucchi:Incyte: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Wondergem:Celgene: Other: Educational talks; Novartis: Other: Educational talks. Pack:Novartis Pharma AG: Current Employment. Wroclawska:Novartis Pharma AG: Current Employment. Wilke:Novartis Pharma AG: Current Employment, Other: Stock owner. Zhang:Novartis Pharma AG: Current Employment. Heidel:Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal